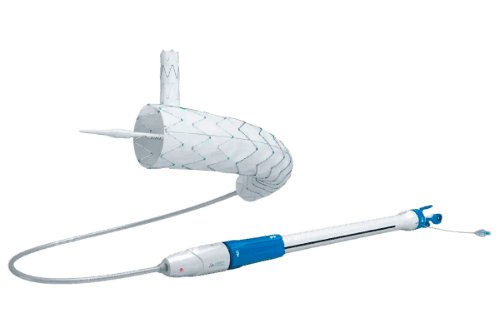
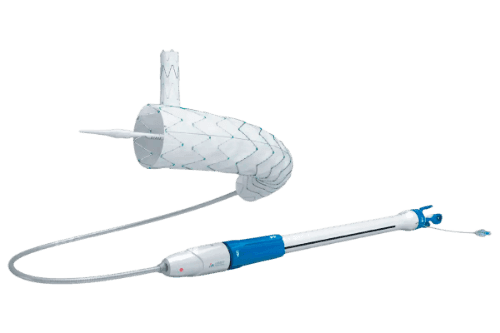
Next level stent design to improve procedural efficiency and patient outcomes
The Cratos™ Thoracic endografts Branch Stent Graft System is a Custom-Made advanced endovascular device offered by Lombard Medical, designed for the treatment of descending thoracic aortic lesions, such as aneurysms, Stanford type B aortic dissections, intramural hematomas (IMH), and penetrating atherosclerotic ulcers (PAU), particularly in cases involving the left subclavian artery (LSA). Building upon the design of its predecessor, the Castor™ stent graft, Cratos™ introduces several delivery system enhancements to improve procedural efficiency and patient outcomes.
Interested in ordering the Cratos stent from Lombard? Get in touch for a quote, clinical brochure, or to arrange a product demo.